APPLICABLE RULES- BIO WASTE MANAGEMENT RULES,2016
DEFINITION – Bio Medical Waste Authorization is a mandatory compliance for all healthcare facilities, including hospitals, clinics, laboratories, veterinary institutions, and blood banks, to ensure safe disposal of infectious and hazardous waste. The authorization is issued under the Bio-Medical Waste Management Rules, 2016, governed by the State Pollution Control Board (SPCB). Proper segregation, collection, and disposal of biomedical waste help prevent environmental contamination and health risks.
Corpzo assists healthcare establishments in obtaining Bio-Medical Waste Authorization seamlessly. Our experts handle documentation, application filing, liaison with SPCB, and compliance monitoring. We ensure your facility meets all environmental standards and waste management norms efficiently. With Corpzo, you can stay focused on healthcare while we manage your environmental compliance responsibilities with complete transparency and professionalism.
Bio Medical Waste means any waste which is generated during the treatment, diagnosis, or immunisation of animals or human beings or research activities pertaining thereto or in the production or testing of Biological products or in health camps, including the categories mentioned in Schedule I.
APPLICABILITY –These rules shall apply to all persons who generate , collect , receive, store , transport, treat , dispose or handle Bio Medical Waste in any form including hospitals , nursing homes , clinics , dispensaries , veterinary institutions , animal houses , pathological laboratories , blood banks , ayush hospitals , clinical establishments , research or educational institutions , health camps, medical or surgical camps , vaccination camps , blood donation camps , first aid rooms of schools , forensic laboratories and research labs .
Non-applicability –
- Radioactive wastes
- Hazardous chemical
- Solid waste covered
- The lead-acid batteries
- Waste cover under E Waste (Management and Handling Rules ) 2011
Obligation of the operator of a common Biomedical waste treatment and disposal facility
- Take all necessary steps to ensure that the Bio medical waste collected from the occupier is transported, handled, stored, treated and disposed of without any adverse effect to the environment and human health, in accordance with these rules and guidelines issued by the central pollution control board and the Central Government, or as the case may be, from time to time.
- Ensure the timely collection of biomedical waste from the occupier as prescribed under these rules
- Establish bar coding and a global positioning system for handling of biomedical waste within one year
- Inform the prescribed authority immediately regarding the occupiers who are not handing over the segregated biomedical waste in accordance with these rules.
- Assist the occupier in training conducted by him for biomedical waste management
Treatment and disposal-
Biomedical waste shall be treated and disposed of in accordance with Schedule 1 and in compliance with standards provided in Schedule II by the health care facilities and the common biomedical waste treatment facility.
Occupier shall deliver segregated waste as per Schedule 1 to the common biomedical waste treatment facility for treatment, processing, and final disposal.
In cases where the services of the common biomedical waste treatment are not available, the occupiers shall set up the requisite biomedical waste treatment equipment, like an incinerator, autoclave, or microwave.
Prescribed authority-The prescribed authority for implementation of these rules shall be the State Pollution Control Board.
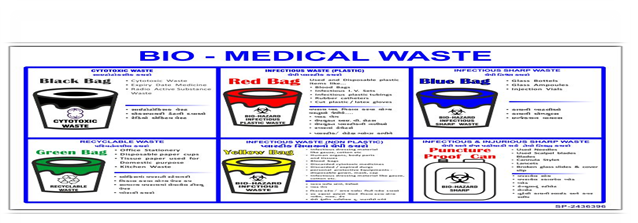
TREATMENT OPTION FOR WASTES
Schedule I
|
Treatment option
|
- Human anatomical waste –Human tissues, ogans, parts, and fetus
- Animal anatomical waste –Experimenta,l animal carcasses, body parts, organs, tissues, including the waste generated from animal use experiments
|
|
|
Yellow colour non-chlorinated plastic bag
|
|
- Soiled waste- items contaminated with blood, body fluids like dressing, plaster casts, cotton swabs, and bags containing residual or discarded blood
|
|
- Expired or discarded medicine- pharmaceutical waste like antibiotics, cytotoxic drugs
|
|
- Chemical waste- chemicals used in the production of Biological products and used or discarded disinfectants
|
|
|
Separate collection system leading to the effluent treatment system
|
- Chemical Liquid waste – liquid waste generated due to the use of chemicals in the production of Biological products and used or discarded disinfectants, silver, and ray.
|
|
|
Non-chlorinated yellow plastic bags or suitable packing material
|
- Discarded Linen , mattresses , beddings contaminated with blood or body fluid
|
|
|
Auto clave safe plastic bags or containers
|
- Micro Biology , Biotechnology and other clinical Laboratory waste
|
|
|
Red colour non chlorinated plastic bags
|
- Contaminated waste (recyclable)
|
|
|
White translucent
|
- Waste sharps including metals
|
|
|
Blue
|
|
|
Procedure for authorisation-
- Create Login on the pollution department portal
For applying applicant needs to register them self on the state pollution board’s web portal
- Apply in form II
Make an application in Form II to State Pollution Control Board, fill up all the details of the unit, all the detail of bio-medical waste generated in the unit and attach the required documents.
- Review and Payment
After filling up all the details in the form, review the form before submission and make the payment of government fees (generally the fees is of 5k but it may vary state to state)
- Application scrutinize by the department
The department will check the application and make sure that the applied application is okay with all described parameter Bio-Medical Waste (Management and Handling) Rules, 1998. In case of denial of renewal , cancellation or suspension of the authorisation by the prescribed authority the reasons shall be recorded in writing
- Issue of License
The authorisation shall be one time for non-bedded occupiers and the authorisation in such cases shall be deemed to have been granted if not objected by the prescribed authority within a period of 90 days from the date of receipt of a duly completed application along with such necessary documents.In case of any change in the Bio-medical waste generation, handling, treatment and disposal for which authorisation was earlier granted the occupier or operator shall inform the prescribed authority about the change or variation in the activity and shall submit a fresh application in form II for modification of the conditions of authorisation.
Documents required-
- Id and proof of address of authorised person of medical establishment
- Pan card of authorised person
- Mcd license
- Agreement of waste collection
- Incorporation documents of company such as Pan Moa /Aoa
- Board declaration for authorised signatory
When License is required
- Prior to establishment
- Prior to expansion of undertaking
When ownership gets changed, Conclusion: Obtaining Bio-Medical Waste Authorization ensures legal compliance and promotes environmental sustainability. Partner with Corpzo for expert guidance and a hassle-free authorization process tailored to your healthcare facility’s needs.
An Overview of Bio-medical Waste Management Authorisation
It is believed that hospitals generate an enormous amount of biomedical waste every year that must be managed effectively. For instance, proper waste management is crucial in maintaining a healthy environment, not just for the hospitals but for the environment on a more significant level. This guide explores various factors related to bio-medical waste management and handling or disposal of bio-medical waste..
It is undeniable how hospitals deal with a wide range of biomedical waste, which requires safe handling and disposal. Failing to comply with the same gives birth to various issues, causing a nuisance to the environment, causing pollution or encouraging any legal troubles. In this guide, we discuss the significance of bio-medical waste management, the categorization of the waste, and the measures adopted in matters of waste management.
Now, whether you are a hospital administrator or a healthcare professional, this guide will fulfil your needs by providing you with the required knowledge and the tools to ensure that the hospital environment is safer and sounder. Let us explore the world of bio-medical waste management and its significance in creating a healthier environment.
What is Meant by Bio-medical Waste Management?
Bio-medical waste is those wastes generated from healthcare activities, which are contagious to many diseases. Like that, the board regulates carrying out the provisions of the Bio-medical Waste Management Rules, 2016, which highlights the norms around the storage, treatment and disposal of BMWs.
The rationale behind Bio-medical waste practice mainly centres on reducing, recycling and reusing waste. It restricts waste generation while managing it better than simply disposing of it.
According to Biomedical Waste Management 2016, "bio-medical waste" means any waste that is generated during the diagnosis, treatment or immunization of human beings or animals or research activities about it or in the production or testing of biological or health camps, including the categories mentioned in Schedule I appended to these rules.
Therefore, efforts have been made to improve the processes of collection, segregation or disposal of bio-medical wastes in an eco-friendly manner, which would eventually minimize the bio-medical waste generation, keeping in view its influence on the environment.
Salient Features of Bio-medical Waste Management
The rules as per the Bio-medical waste management apply to the persons, who generate, collect, receive, and store bio-medical waste in various forms including clinics, nursing homes, dispensaries and pathological laboratories, research, clinical establishments, educational institutions, medical and surgical camps with forensic laboratories.
It is widely acknowledged that the establishments undertaking battery waste management must seek necessary permissions and authorizations, which are primarily meant for the non-bedded occupiers, with which the Biomedical Waste Authorisation puts forth the needed requirements or the procedures, which mainly highlight
In obtaining the authorisation, the respective HCEs falling within these rules must submit the Annual-returns on the records of the biomedical waste generation along with the disposal to the State Board by 30th June each year as per the prescribed form IV from the Bio-medical Waste management rules, 2016.
With the provision of these rules, it becomes essential for the HCEs to be able to treat the Bio-medical waste management generation via the authorised facility of Bio-medical waste treatment facility (CBWTF)
The duties and responsibilities are no less than the necessary steps that ensure that biomedical waste management is undertaken well and is handled without any negative effect on the environment and human health along with the rules, that mention
Duties of the Authorities
It mentions a provision meant for the safe, secured and ventilated location for the storage of the segregation of biomedical waste, especially in the containers or the coloured bags in the manner specified as per Schedule I, to make sure there are no forms of secondary handling, pilferage or recyclables or spillage by animals as well as the bio-medical waste form such a place/ premises that needs to be transported as per those rules prevailing to the bio-medical waste from a place that is transported in a manner with the biomedical waste treatment facility with the adequate form of treatment or disposal;
To pre-treat the laboratory waste, microbiological waste and blood bags via disinfection or sterilisation on-site as per the manner prescribed by World Health Organisation (WHO) or National AIDs control organisation ( NACO) guidelines, which is then forwarded to the common bio-medical waste treatment facility for the means to final disposal
t demands them to phase out chlorinated plastic bags, blood bags, along gloves within two years from the date of the notification of these major rules
Disposal of solid waste, besides the bio-medical waste following the provisions of the respective waste management rules that are established as per the relevant laws passed on from time to time
It does not give the treated bio-medical waste with the municipal solid waste.
Therefore, it does not end here, but there are many other rules for treating Bio-medical waste management.
Duties of the operator
It is the duty of every operator
To undertake the vital step in ensuring that the bio-medical waste gathered from the occupier is transported, stored handled and disposed of, without having any negative effects on the environment or human health based on the guidelines and rules issued by the Central Government, the central pollution control board promptly.
It is to ensure the collection of the bio-medical waste from the occupier as per the mentioned rules
It is vital to inform the respective authorities, regarding the occupier that does not handle the segregated bio-medical waste based on the rules and regulations.
To provide the required training to the workers involved in the handling of bio-medical waste, during the induction, at least once a year;
To undertake the medical examination during the indiction or maybe once a year to immunise the workers with the tasks of handling the bio-medical waste from any diseases, such as Hepatitis B and tetanus, which are transmitted easily, while handling the bio-medical waste, while maintaining the records for the same.
Step-by-Step Procedure for Bio-medical Waste Management Authorisation
The step-by-step procedure to achieve the bio-medical waste management Authorisation is as follows
Contact Corpzo
First, contact Corpzo as your one-stop solution provider.
Corpzo aligns a dedicated professional
Secondly, Corpzo aligns a dedicated professional to help you with Biomedical waste management
Filling out the Application
In the third step, we help you fill out the application as stated in the Form II, which will be submitted by us on your behalf to the concerned authority. It is a crucial step in encouraging you to attain the authorisation.
Authorisation from the authority
In the following step, the authority will perform its role of granting you with the authorisation as per Form III, which includes mainly the validity of the authorisation for the healthcare facility and also with the operator of the common facility having consent validity.
Granted Authorisation
You need to note that the authorisation is mainly for the non-bedded occupiers and in all these cases, it has been granted, and not forced by the authority within a span of ninety days, including the date of receipt of the required application found to be attached with the respective documents. Note- In the cases of the refusal/Renewal or cancellation of authorisation from the main authority, the reasons stemming from the same will have to be recorded in writing, only after the permissions from the authority, which would allow the applicant to be heard before any authorisation is granted.
Therefore, you need not worry about the hassle, as you have the right guide to encourage or assist you with Biomedical waste management.
Effortless Bio-medical Waste Management Authorisation with Corpzo by Your Side
At Corpzo, we deeply value the immense impact of businesses in India. Our Unwavering commitment to ensuring smooth operations and long-term success is rooted in our understanding of the crucial role regulations and requirements play in achieving these goals. We recognise the complexities and challenges entrepreneurs face when navigating the legal landscape and are dedicated to providing comprehensive guidance and support. With our team of experienced experts, we help our clients obtain accreditation, enabling them to achieve their full potential. .